Prediction Cell Parameter of A2MX6 Cubic Perovskites
Main Article Content
Abstract
A2MX6 perovskites materials have shown impressive advances in the last 50 years due on their photovoltaic application, made them one of the most-promising technologies for next-generation solar cells. We present in this work a semi empirical model for prediction of cell parameter of cubic A2MX6 perovskites. It is useful for providing the predicted structural information for estimating the physical properties of materials for which accurate structural data are not available. We propose a linear formula according to the factors: Interatomic distance RA + RX and RM + RX and electronegativity difference 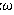
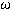 . The interatomic distances dX-X and dM-Xd can be estimated from the crystalline structure of the compounds studied. A new prediction of cell parameter is estimated for 90 perovskites and the results are compared with the experimental one whose error is of the order of 1.13%.
. The interatomic distances dX-X and dM-Xd can be estimated from the crystalline structure of the compounds studied. A new prediction of cell parameter is estimated for 90 perovskites and the results are compared with the experimental one whose error is of the order of 1.13%.
Article Details
Section

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
-
Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
-
ShareAlike — If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original.
- No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.
How to Cite
References
Abriel, W. (1984). Polymorphism and phase transitions of K2TeBr6: A single crystal investigation of the high temperature phases (383–463 K). Materials research bulletin, 19(3), 313-318.. https://doi.org/10.1016/0025-5408(84)90172-7
Abriel, W. & Du Bois, A. (1989). Structure of bis (tetraphenylarsonium) hexabromotellurate (IV). Acta Crystallographica Section C : Crystal Structure Communications, 45(12), 2002-2003. https://doi.org/10.1107/S0108270189008826
Abriel, W. & Ihringer, J. (1984). Crystal structures and phase transition of Rb2TeBr6 (300-12.5 K). Journal of Solid State Chemistry, 52(3), 274-280. https://doi.org/10.1016/0022-4596(84)90010-0
Abriel, W. & White, M. A. (1990). The phase transitions of ferroelastic K2SeBr6: A calorimetric and x-ray diffraction study. The Journal of chemical physics, 93(11), 8321-8327. https://doi.org/10.1063/1.459315
Bagnall, K. W., d'Eye, R. W. M. & Freeman, J. H. (1955). The polonium halides. Part II. bromides. Journal of the Chemical Society (Resumed), 3959-3963. https://doi.org/10.1039/JR9550003959
Bagnall, K. W., D'Eye, R. W. M. & Freeman, J. H. (1956). 657. The polonium halides. Part III. Polonium tetraiodide. Journal of the Chemical Society (Resumed), 3385-3389. https://doi.org/10.1039/JR9560003385
Beck, J. & Hengstmann, M. (1998). Über Thallium (l)-chlorooxomolybdate: Synthese und Kristallstrukturen von Tl [MoOCl4 (NCCH3)], Tl [Mo2O2Cl7] und Tl2 [ Mo4O4Cl14] und die Struktur von Tl2 [MoCl6]. Zeitschrift fur anorganische und allgemeine Chemie, 624(12), 1943-1950. https://doi.org/10.1002/(SICI)1521-3749(1998120)624:12%3C1943::AID-ZAAC1943%3E3.0.CO;2-6
Bland, J. A. & Flengas, S. N. (1961). The Crystal Structure of K2TiCl6. Canadian Journal of Physics, 39(6), 941-944.https://doi.org/10.1139/p61-100
Bode, H. & Voss, E. (1956). Üder strukturen von Fluorokomplexen mit vierwertigem Nickel und Chrom. Zeitschrift für anorganische und allgemeine Chemie, 286(3-4), 136-141. https://doi.org/10.1002/zaac.19562860304
Bork, M. & Hoppe, R. (1996). Der erste Vertreter des K2PtCl6-Typs bei Fluoriden A2 [PtF6]: B-Cs2 [PtF6]. Zeitschrift für anorganische und allgemeine Chemie, 622(3), 417-424. https://doi.org/10.1002/zaac.19966220307
Boysen, H. A. N. S. & Hewat, A. W. (1978). A neutron powder investigation of the structural changes in K2SnCl6. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 34(5), 1412-1418. https://doi.org/10.1107/S0567740878005816
Brill, T. B., Gearhart, R. C. & Welsh, W. A. (1974). Crystal structures of M2SnCl6 salts. An analysis of the “crystal field effect” in their nuclear quadrupole resonance and vibrational spectra. Journal of Magnetic Resonance (1969), 13(1), 27-37. https://doi.org/10.1016/0022-2364(74)90101-2
Brik, M. G. & Kityk, I. V. (2011). Modeling of lattice constant and their relations with ionic radii and electronegativity of constituting ions of A2XY6 cubic crystals (A= K, Cs, Rb, Tl; X= tetravalent cation, Y= F, Cl, Br, I). Journal of Physics and Chemistry of Solids, 72(11), 1256-1260. https://doi.org/10.1016/j.jpcs.2011.07.016
Chen, M., Ju, M. G., Carl, A. D., Zong, Y., Grimm, R. L., Gu, J., ... & Padture, N. P. (2018). Cesium titanium (IV) bromide thin films based stable lead-free perovskite solar cells. Joule, 2(3), 558-570.https://www.cell.com/joule/fulltext/S2542-4351(18)30037-0
Chonghe, L., Yihao, T., Yingzhi, Z., Chunmei, W. & Ping, W. (2003). Prediction of lattice constant in perovskites of GdFeO3 structure. Journal of Physics and Chemistry of Solids, 64(11), 2147-2156. https://doi.org/10.1016/S0022-3697(03)00209-9
Coll, R. K., Fergusson, S. E., Penfold, B. R., Rankin, D. A. & Robinson, W. T. (1990). The preparative and structural chemistries of hexahalogeno and trichlorostannato complexes of iridium. Inorganica chimica acta, 177(1), 107-114. https://doi.org/10.1016/S0020-1693(00)91918-2
Deloume, J. P., Faure, R. & Thomas-David, G. (1979). Nouvelle détermination de la structure cristalline du u-oxo-bis [pentachlororuthénate (IV)] de potassium, K4 [Ru2Cl10O] et affinement de la structure de l'hexachlororuthénate de potassium K2 [RuCl6]. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 35(3), 558-561.https://doi.org/10.1107/S0567740879004143
Edwards, A. J., Peacock, R. D. & Said, A. (1962). 894. Complex chlorides and bromides of quadrivalent molybdenum. Journal of the Chemical Society (Resumed), 4643-4648. https://doi.org/10.1039/JR9620004643.
Elder, M., Fergusson, J. E., Gainsford, G. J., Hickford, J. H. & Penfold, B. R. (1967). Potassium pentachlorohydroxytechnetate (IV). Journal of the Chemical Society A: Inorganic, Physical, Theoretical, 1423-1425. https://doi.org/10.1039/J19670001423
Engel, G. (1935). Die kristallstrukturen einiger hexachlorokomplexsalze. Zeitschrift für Kristallographie. Crystalline Materials, 90(1-6), 341-373. https://doi.org/10.1524/zkri.1935.90.1.341
Feng, L. M., Jiang, L. Q., Zhu, M., Liu, H. B., Zhou, X. & Li, C. H. (2008). Formability of ABO3 cubic perovskites. Journal of Physics and Chemistry of Solids, 69(4), 967-974. https://doi.org/10.1016/j.jpcs.2007.11.007
Ferrari, A. & Coghi, L. (1941). On the existence of hexahalogenoaurates. Gazz. Chim. Ital, 71, 440-441 https://doi.org/10.1021/ic50081a044
Fukunaga, O. & Fujita, T. (1973). The relation between ionic radii and cell volumes in the perovskite compounds. Journal of Solid State Chemistry, 8(4), 331-338. https://doi.org/10.1016/S0022-4596(73)80030-1
Geiger, E. (1926). Ueber die Konstitution der Hochpolymeren (Doctoral dissertation, ETH Zurich). https://doi.org/10.1016/j.jpcs.2011.07.016
Grundy, H. D. & Brown, I. D. (1970). A refinement of the crystal structures of K2ReCl6, K2ReBr6, and K2PtBr6. Canadian Journal of Chemistry, 48(7), 1151-1154. https://doi.org/10.1139/v70-189
Henke, H. (2007). The significance of the Jahn-Teller effect for the phase transitions of K2NbCl6 and Rb2NbCl6. Zeitschrift für Kristallographie, 222(9), 477-486. https://doi.org/10.1524/zkri.2007.222.9.477
Hester, J. R., Maslen, E. N., Spadaccini, N., Ishizawa, N. & Satow, Y. (1993). Accurate synchrotron radiation Ap maps for K2SiF6 and K2PdCl6. Acta Crystallographica Section B: Structural Science, 49(6), 967-973. https://doi.org/10.1107/S0108768193005725
Hoppe, R. & Hofmann, B. (1977). News on K2(MnF6), Rb2/(MnF6) and Cs2/(MnF6). Z. Anorg. Allg. Chem, 436. 65-74. https://inis.iaea.org/records/5ns1n-g4r88
Hoppe, R. & Klemm, W. (1952). Über Fluorokomplexe des Palladiums und des Goldes. Zeitschrift für anorganische und allgemeine Chemie, 268(4-6), 364-371. https://doi.org/10.1002/zaac.19522680415
Hu, B., Wang, P., Xiao, Y. & Song, L. P. (2005). Crystal structure of dicesium hexachloromolybdate (IV), Cs2 [MoCl6]. Zeitschrift für Kristallographie-New Crystal Structures, 220(1-4), 318-318. https://doi.org/10.1524/ncrs.2005.220.14.318
Jiang, L. Q., Guo, J. K., Liu, H. B., Zhu, M., Zhou, X., Wu, P. & Li, C. H. (2006). Prediction of lattice constant in cubic perovskites. Journal of Physics and Chemistry of Solids, 67(7), 1531-1536. https://doi.org/10.1016/j.jpcs.2006.02.004
Jongen, L. & Meyer, G. (2004). Dipotassium hexachlorotantalate (IV), K2TaCl6. Acta Crystallographica Section E: Structure Reports Online, 60(8), 91-92. https://doi.org/10.1107/S1600536804016198
Kalaiselvi, C. R., Muthukumarasamy, N., Velauthapillai, D., Kang, M. & Senthil, T. S. (2018). Importance of halide perovskites for next generation solar cells–a review. Materials Letters, 219, 198-200. https://doi.org/10.1016/j.matlet.2018.02.089
Ketelaar, J. A. A. (1935). Die Kristallstruktur von K-, Rb-, Cs-und Tl-Silicofluorid und von LiMnO4-3H2O. Zeitschrift für Kristallographie-Crystalline Materials, 92(1-6), 155-156. https://doi.org/10.1524/zkri.1935.92.1.155
Ketelaar, J. A. A., Rietdijk, A. A. & Van Staveren, C. H. (1937). Die Kristallstruktur von Ammonium-, Kalium-, Rubidium-und Cäsiumstannibromid. Recueil des travaux Chimiques des Pays-bas, 56(9), 907-908. https://doi.org/10.1002/recl.19370560913
Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. (2009). Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. Journal of the American chemical society, 131(17), 6050-6051. https://pubs.acs.org/doi/10.1021/ja809598r
Kumar, A., Verma, A. S. & Bhardwaj, S. R. (2008). Prediction of formability in perovskite-type oxides. The Open Applied Physics Journal, 1:11-19. http://dx.doi.org/10.2174/1874183500801010011
Lalancette, R. A., Elliott, N. & Bernal, I. (1972). Crystal structures and magnetic properties of a 2 mncl 6 salts (a= nh4+, k+ and rb+). Journal of Crystal and Molecular Structure, 2, 143-149. https://doi.org/10.1007/BF01464795
Laubengayer, A. W., Billings, O. B. & Newkirk, A. E. (1940). Chlorogermanic acid and the chlorogermanates. Properties and crystal structure of cesium hexachlorogermanate. Journal of the American Chemical Society, 62(3), 546-548. https://pubs.acs.org/doi/pdf/10.1021/ja01860a027.
Lee, J. W. & Cho, J. S. (2010). Effective lane detection and tracking method using statistical modeling of color and lane edge-orientation in Advanced in Information Sciences and Service Sciences. DOI:10.4156/aiss.vol2.issue3.16Source:DBLP
Lide, D. R. (Ed.). (2004). CRC handbook of chemistry and physics (Vol. 85). CRCpress. https://journals.iucr.org/paper?pii=S0567739476001551
Lufaso, M. W. (2002). Perovskite synthesis and analysis using structure prediction diagnostic software (Doctoral dissertation, The Ohio State University). https://www.proquest.com/openview/837fefb7f3c7154a3c068d24795383e5/1?pq-origsite=gscholar&cbl=18750&diss=y
Lufaso, M. W. & Woodward, P. M. (2001). Prediction of the crystal structures of perovskites using the software program SPuDS. Acta Crystallographica Section B: Structural Science, 57(6), 725-738. https://doi.org/10.1107/S0108768101015282
Majid, A., Khan, A. & Choi, T. S. (2011). Predicting lattice constant of complex cubic perovskites using computational intelligence. Computational Materials Science, 50(6), 1879-1888. https://doi.org/10.1016/j.commatsci.2011.01.035
Magette, M. & Fuger, J. (1977). Preparation, crystal structure and enthalpy of formation of dicaesium hexabromoneptunate. Inorganic and Nuclear Chemistry Letters, 13(10), 529-536. https://doi.org/10.1016/0020-1650(77)80023-8
Maughan, A. E., Ganose, A. M., Bordelon, M. M., Miller, E. M., Scanlon, D. O. & Neilson, J. R. (2016). Defect tolerance to intolerance in the vacancy-ordered double perovskite semiconductors Cs2SnI6 and Cs2TeI6. Journal of the American Chemical Society, 138(27), 8453-8464. https://pubs.acs.org/doi/10.1021/jacs.6b03207
McCullough, J. D. (1936). Kristallgeometrie Kristallphys Kristallchem. Z Kristallogr, 94, 143. https://doi.org/10.1524/zkri.1936.94.1.143
Moreira, R. L. & Dias, A. (2007). Comment on “Prediction of lattice constant in cubic perovskites”. Journal of Physics and Chemistry of Solids, 68(8), 1617-1622. https://doi.org/10.1016/j.jpcs.2007.03.050
Mows, P.C. (1966). The Crystal Structure, Visible, and Ultraviolet Spectra of Potassium Hexachloromanganate (IV). Inorganic Chemistry, 5, 5-8. https://doi.org/10.1021/ic50035a002
Nagorny, S. (2021). Novel Cs2HfCl6 Crystal Scintillator: Recent Progress and Perspectives, Physics 2021, 3(2), 320-351. https://doi.org/10.3390/physics3020023
Saalfeld, H. & Guse, W. (1983). The polymorphism of K2HfF6. https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCAL83X0323830
Schüpp, B., heines, P. & keller, H. L. (2000). Zwei neue lodopalladate mit hleicher Summenformel: Rb2Pdl4. L2-ein neuer Strukturtyp mit eingelagerten l2-Molekülen-und Rb2Pdl6. Zeitschrift für anorganische und allgemeine Chemie, 626(1), 202-207. https://doi.org/10.1002/(SICI)1521-3749(200001)626:1%3C202::AID-ZAAC202%3E3.0.CO;2-7
Schüpp, B., Heines, P., Savin, A. & Keller, H. L. (2000). Crystal Structures and Pressure-Induced Redox Reaction of Cs2PdI4I2 to Cs2PdI6. Inorganic Chemistry, 39(4), 732-735. https://doi.org/10.1021/ic990670
Schütz, W. (1936). Die kristallchemische Verwandtschaft zwischen Germanium und Silicium. Zeitschrift für Physikalische Chemie, 31(1), 292- 308. https://doi.org/10.1152/physrev.1957.37.4.475
Shannon, R. D. (1976). Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Foundations of Crystallography, 32(5), 751-767. https://doi.org/10.1107/S0567739476001551
Sinram, D., Brendel, C. & Krebs, B. (1982). Hexa-iodoanions of titanium, zirconium, hafnium, palladium and platinum: preparation, properties and crystal structures of the caesium salts. Inorganica Chimica Acta, 64, L131-L132. 10.1016/s0020-1693(00)90301-3
Smith, P. J., White, R. F. M. & Smith, L. (1972). A 119Sn NMR and Mössbauer study of some di-and tri-alkyltin (IV) alkoxides. Journal of Organometallic Chemistry, 40(2), 341-353. https://doi.org/10.1080/00958970500240649
Smok, P., Kityk, I. V., Plucinski, K. J. & Berdowski, J. (2002). Origin of giant anisotropy in synthesized Ba pentaborates. Physical Review B, 65(20), 205103. https://doi.org/10.1103/PhysRevB.65.205103
Sperka, G. & Maunter, F. A. (1988). Crystal growth and structure of Cs2ReCl6. Crystal research and technology, 23(7), K109-K111. https://doi.org/10.1515/znb-1983-0802
Stoll, P. (1926). Raumgitter von Komplexsalzen (Doctoral dissertation, ETH Zurich).
Takazawa, H., Ohba, S. & Saito, Y. (1988). Electron-density distribution in crystals of dipotassium tetrachloropalladate (II) and dipotassium hexachloropalladate (IV), K2 [PdCl4] and K2 [PdCl6] at 120 K. Acta Crystallographica Section B: Structural Science, 44(6), 580-585. https://doi.org/10.1107/S0108768188010031
Taylor, J. C. (1987). A comparison of profile decomposition and Rietveld methods for structural refinement with powder diffraction data. Zeitschrift für Kristallographie-Crystalline Materials, 181(1-4), 151-160. https://doi.org/10.1524/zkri.1987.181.14.151
Thiele, G., Mrozek, C., Kammerer, D. & Wittmann, K. (1983). On hexaiodoplatinates (iv) m2pti6 (m= k, rb, cs, nh4, tl)-preparation, properties and structural data. Zeitschrift Fur Naturforschung Section Ba Journal of Chemical Sciences, 38(8), 905-910. https://doi.org/10.1515/znb-1983-0802
Vdovenko, V. M., Kozhina, I. I., Suglobova, I. G. & Chirkst, D. E. (1973). Complexing in uranium halogenide-alkali metal halogenide systems. Radiokhimiya, 15(1), 54-57 https://inis.iaea.org/search/search.aspx?orig_q=RN:407345957
Collins, P. H. & Webster, M. (1974). Crystal and molecular structure of tetraphenylarsonium aquotetrachlorohydroxotellurate (IV). J. Chem. Soc., Dalton Trans. (15). https://doi.org/10.1039/DT9740001545.
Wei, D. Y., Lin, J. L. & Zheng, Y. Q. (2002). Note: The crystal structure of Tris (nitrato-O, O') bis (1, 10-phenanthroline-N, N') terbium (III). Journal of Coordination Chemistry, 55(11), 1259-1262.
Werker, W. (1939). Die krystallstruktur des Rb2SnJ6 und Cs2SnJ6. Recueil des Travaux Chimiques des Pays-Bas, 58(3), 257-258. https://doi.org/10.1002/recl.19390580309
Williams, R. J., Dillin, D. R. & Milligan, W. O. (1973). Structure refinement of potassium chloroplatinate by powder and single-crystal methods. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 29(7), 1369-1372. https://doi.org/10.1107/S0108768188010031
Wyckoff, R. W. & Müller, J. H. (1927). The crystal structure of cesium fluogermanate. Am J Sci, 76, 347-352. https://ajsonline.org/cgi/doi/10.2475/ajs.s5-16.94.349
Xu, R., Ye, Y. & Zhao, W. (Eds.). (2012). Introduction to natural products chemistry (pp. 101-118). Science press. DOI : 10.1524/zkri.217.3.301.16032
Yang, W. S., Park, B. W., Jung, E. H., Jeon, N. J., Kim, Y. C., Lee, D. U., ... & Seok, S. I. (2017). Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science, 356(6345), 1376-1379. https://doi.org/10.1126/science.aan2301
Yao, J., Kong, J., Shi, W. & Lu, C. (2022). The insolubility problem of organic hole-transport materials solved by solvothermal technology: toward solution-processable perovskite solar cells. ACS Applied Materials & Interfaces, 14(5), 7493-7503. https://doi.org/10.1021/acsami.1c24035
Yun, H. & Jang, G. J. (2007). Dicaesium hexachlorotantalate (IV), Cs2TaCl6. Acta Crystallographica Section E: Structure Reports Online, 63(1), i22-i23. https://doi.org/10.1107/S1600536806054754
Zheng, Y. Q., Nuss, J. & Schnering, H. V. (1998). Crystal structure of dithallium hexachlorotungstate (IV), Tl2 [WCl6]. Zeitschrift für Kristallographie-New Crystal Structures, 213(1-4), 500-500 https://doi.org/10.1524/ncrs.1998.213.14.500.
Zheng, Y. Q., Peters, K. & Schnering, H. V. (1997a). Crystal structure of dirubidium hexabromotungstate (IV), Rb2WBr6. Zeitschrift für Kristallographie-Crystalline Materials, 212(1), 53-53. https://doi.org/10.1515/zkri-1997-2120117.
Zheng, Y. Q., Peters, K. & Schnering, H. V. (1997b). Crystal structure of dicesium hexabromotungstate (IV), Cs2WBr6. Zeitschrift für Kristallographie-Crystalline Materials, 212(1), 55-55. https://doi.org/10.1515/zkri-1997-2120119.
Zheng, Y. Q., Sun, J. & Wang, Y. F. (2005). The first suberato-bridged lanthanide coordination polymer: synthesis, crystal structure and properties of La2 (H2O) 2L3 (H2L= HOOC (CH2) 6COOH). Journal of Coordination Chemistry, 58(17), 1551-1559. https://doi.org/10.1016/S0022-328X(00)93372-2.




